Contrary to popular belief, the U.S. Food and Drug Administration (FDA)The federal agency responsible for regulating food, drugs, medical devices, and other products to ensure safety and efficacy. does not have the legal power to mandate drug recall actions. Instead, the agency can only request that manufacturers voluntarily remove unsafe medications. This key distinction is often misunderstood, even by healthcare professionals. The Federal Food, Drug, and Cosmetic Act (FD&C Act)The primary law governing the FDA's authority over drugs, medical devices, and food safety, enacted in 1938. (FD&C Act) is the foundation of FDA regulations, but it specifically limits the agency's recall authority for drugs.
The FDA's role in drug recalls is primarily advisory. When safety issues arise, the agency works with manufacturers to initiate a voluntary recall. If a company refuses, the FDA must seek legal remedies through court injunctions under Section 304 of the FD&C Act. This process can take weeks or months, creating dangerous delays. For example, during the 2018 valsartan contamination crisis, Chinese API manufacturers delayed cooperation for 17 days, prolonging the recall process despite FDA alerts. The agency's limited power has drawn criticism from public health experts who argue it creates unnecessary risks for patients.
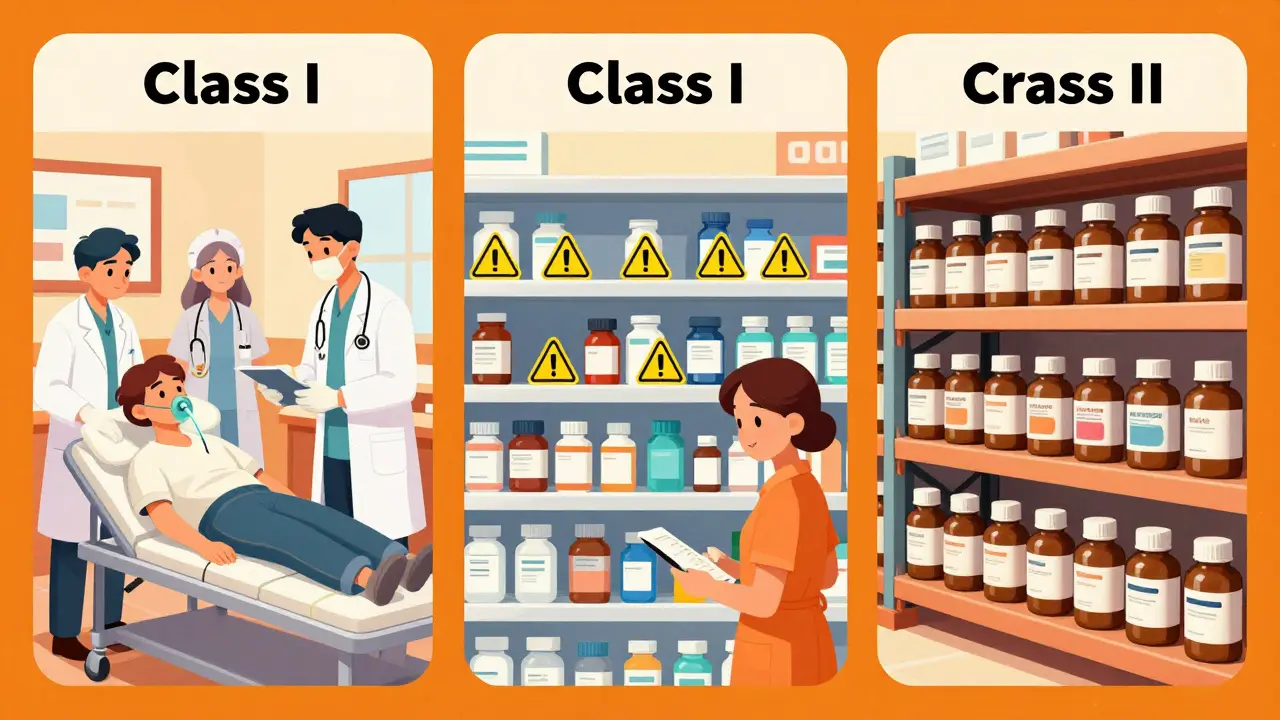
The Three Classes of Drug Recalls
Recalls are categorized based on severity under 21 CFR Part 7The FDA regulation that defines recall classifications and procedures for drugs and medical products. guidelines. This classification system helps determine how quickly and broadly a recall must be implemented.
| Recall Class | Description | Severity Level |
|---|---|---|
| Class I | A reasonable probability that the product will cause serious health consequences or death | Most severe |
| Class II | Products that may cause temporary or reversible health issues, or where serious harm is unlikely | Moderate severity |
| Class III | Products unlikely to cause harm but violate labeling or manufacturing standards | Least severe |
According to FDA data from 2022, Class I recalls make up just 2.1% of all drug recalls, while Class II accounts for 68.7% and Class III for 29.2%. This shows most recalls are for non-critical issues like labeling errors or minor contamination. However, even Class I recalls can take time. The FDA now requires manufacturers to act within 24 hours for Class I recalls, down from the previous 72-hour standard in 2010. This change came after experts highlighted how delays in high-risk recalls endanger patient lives.
The Recall Process Step by Step
When a drug recall begins, the process follows strict procedures. Here's how it typically unfolds:
- Discovery: Issues may be found during manufacturer stability testing (required annually) or through FDA surveillance systems like MedWatchThe FDA's adverse event reporting program that collects over 1.2 million reports annually..
- Notification: The manufacturer notifies the FDA immediately and develops a Recall Strategy document detailing the scope, notification methods, and retrieval steps.
- Depth determination: The FDA and manufacturer decide how far the recall extends-whether to patients, pharmacies, or distributors. Class I recalls usually require patient-level notification.
- Implementation: The manufacturer issues public alerts, contacts distributors, and retrieves affected products. Hospitals and pharmacies must check their inventory against recall lists.
- Verification: The FDA monitors the recall's effectiveness and may require follow-up reports.
Real-world challenges exist. A 2022 ASHP survey found 68% of hospital pharmacy directors struggle to identify affected products due to inconsistent lot numbering systems across manufacturers. This causes delays in patient notification, with some Class I recalls taking 3.7 days longer than necessary. In one case, a contaminated heart medication recall led to 14 avoidable hospitalizations because pharmacies couldn't quickly match affected batches.
Drug vs. Medical Device Recalls: A Critical Difference
There's a major regulatory gap between drugs and medical devices. While the FDA can only request drug recalls, it has mandatory recall authorityFor medical devices under 21 CFR 810 when there's a risk of serious health consequences. for devices. Under 21 CFR 810, the FDA can order a device recall if there's a reasonable probability of serious harm. This difference stems from the Medical Device Amendments of 1976, which granted stronger enforcement powers for devices than the original 1938 FD&C Act for drugs.
For example, when a faulty pacemaker was discovered in 2021, the FDA immediately issued a mandatory recall order under 21 CFR 810. The manufacturer had to remove all affected units within 72 hours. In contrast, similar issues with drugs often rely on voluntary cooperation, leading to longer delays. This regulatory asymmetry has been a point of contention for years, with critics arguing drug safety needs stronger oversight.
Real-World Example: The 2018 Valsartan Recall
The valsartan recall illustrates the complexities of drug recalls. In June 2018, the FDA discovered NDMA, a carcinogen, in blood pressure medication made by several manufacturers. The agency issued public alerts on June 8, 2018, and within 21 days, major U.S. companies had voluntarily recalled affected lots. However, Chinese API manufacturers-responsible for the active ingredient-took 17 days to respond due to communication gaps. This delay highlighted systemic issues in global supply chains. By August 2018, all affected products were removed from the market, but the incident exposed how voluntary recall systems can fail without stronger enforcement tools. The FDA later implemented stricter foreign facility inspections after this case.

Recent Changes and Future Trends
Regulatory changes are ongoing. The FDA's September 2023 guidance updated Class I recall timelines from 72 hours to 24 hours for manufacturers. However, experts argue more is needed. Dr. Peter Lurie of the Center for Science in the Public Interest states: "The FDA's inability to order mandatory drug recalls remains a critical vulnerability, especially for complex biologics where contamination risks are increasing." Meanwhile, PhRMA argues voluntary recalls work well-only 3 out of 15,241 drug recalls required FDA enforcement between 2012-2022.
The agency has also increased inspections of foreign manufacturing facilities, particularly in India and China, where 60% of active pharmaceutical ingredients are produced. This effort aims to prevent contamination issues like the valsartan case. However, the FDA's budget for drug safety oversight has not kept pace with the growing complexity of global supply chains. Proposed legislation like the PREVENT Pandemics Act (S.2871) includes Section 3103, which would grant the FDA explicit mandatory recall authority for drugs. Industry lobbying against this provision has been intense, with PhRMA spending $8.2 million in Q2 2023 to oppose it. As global drug supply chains grow more complex, the debate over FDA recall authority continues to shape patient safety.
Frequently Asked Questions
Can the FDA force a drug recall?
No. The FDA can only request voluntary recalls under the FD&C Act. For drugs, the agency must seek court injunctions to stop distribution if a manufacturer refuses. This is different from medical devices, where the FDA can issue mandatory recalls under 21 CFR 810.
What are the different recall classes for drugs?
Class I recalls involve products that could cause serious harm or death. Class II recalls are for products that may cause temporary or reversible issues. Class III recalls are for non-harmful violations like labeling errors. In 2022, 2.1% of recalls were Class I, 68.7% Class II, and 29.2% Class III.
How does the FDA find unsafe drugs?
The FDA uses multiple systems: manufacturer stability testing (required annually), the MedWatch program (which received 1.2 million adverse event reports in 2022), and inspections of manufacturing facilities. Healthcare providers also report issues through the MedWatch system.
What happens if a company refuses a recall?
The FDA must pursue legal action through court injunctions under Section 304 of the FD&C Act. This process can take weeks or months. For example, in the valsartan case, Chinese manufacturers delayed cooperation for 17 days before complying with FDA requests.
How long does a drug recall take?
It varies. Class I recalls now require action within 24 hours of FDA notification. However, the entire process-including notification, retrieval, and verification-can take weeks. In 2022, 68% of hospitals reported delays in identifying affected products due to inconsistent lot numbering.
Are drug recalls the same as medical device recalls?
No. Drug recalls rely on voluntary manufacturer cooperation, while the FDA can mandate device recalls under 21 CFR 810. This difference stems from how the FD&C Act and Medical Device Amendments of 1976 were written, giving stronger enforcement powers for devices.
What role do hospitals play in drug recalls?
Hospitals must quickly identify affected medications in their inventory, notify patients, and return recalled products. ASHP guidelines require hospitals to designate a recall coordinator, maintain a centralized database of recalled drugs, and train staff monthly on recall procedures. Despite these protocols, 42% of hospitals experienced communication breakdowns during Class I recalls in 2022.





Write a comment
Your email address will be restricted to us