Generic Drug Savings Calculator
Enter the price of your brand-name prescription drug to see how much you could save with a generic alternative.
Estimated Savings
Note: This calculator uses an average 85% savings rate for generic drugs. Most generics cost 80-85% less than brand names. The actual savings may vary. For narrow therapeutic index drugs like warfarin or levothyroxine, always consult your doctor before switching manufacturers.
When you pick up a prescription, you might see two very different bottles on the counter. One says Prilosec, costs $150, and has a bright purple capsule. The other says omeprazole, costs $4, and is a small white pill. You might wonder: Is this cheaper version any good? Does it even work the same?
The short answer is yes - for nearly every drug, it does. But the differences in how they look and what’s written on the label can make you question that. The truth is, the U.S. Food and Drug Administration (FDA) has spent decades making sure generic drugs aren’t just cheaper copies. They’re exact therapeutic matches. The only real differences are in packaging, color, and price.
What Makes a Generic Drug a Generic Drug?
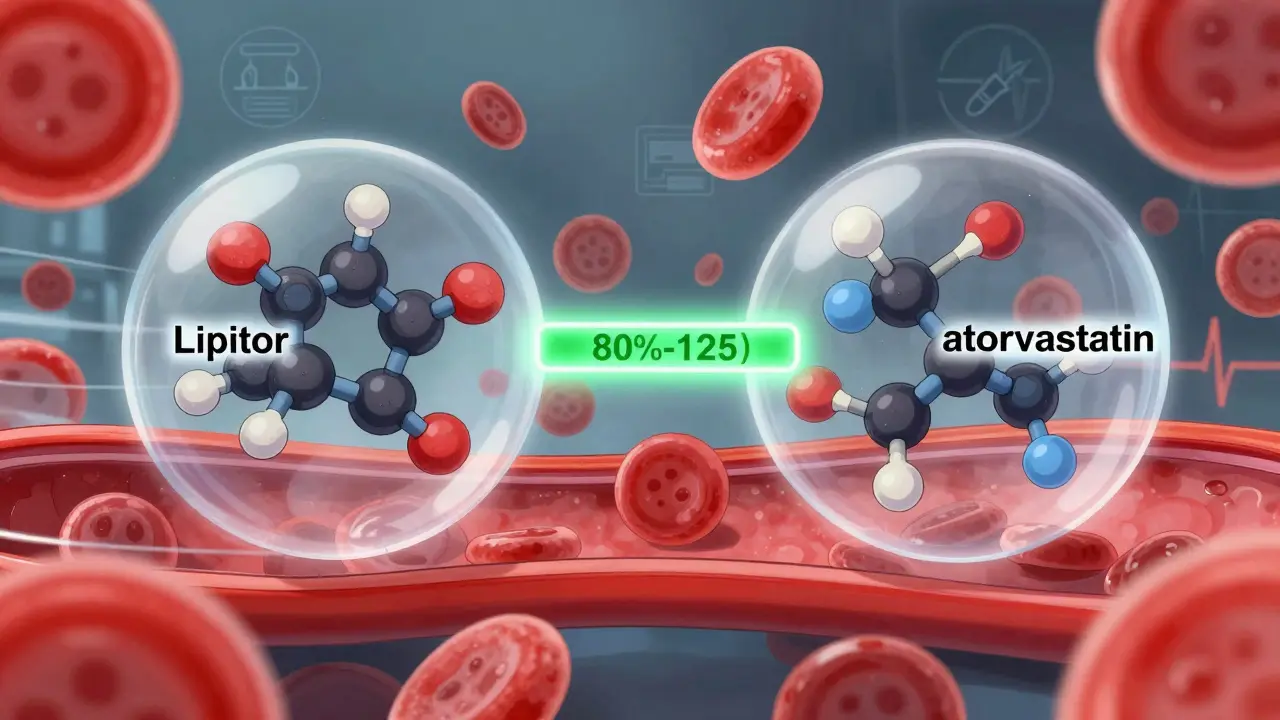
A generic drug isn’t a knockoff. It’s not a weaker version. It’s a pharmaceutical twin. The FDA requires that every generic drug contain the same active ingredient, in the same amount, and delivered the same way as the brand-name version. If your brand-name drug is 20 mg of atorvastatin taken once daily by mouth, the generic must be exactly that - no more, no less.
The FDA’s approval process for generics is called the Abbreviated New Drug Application (ANDA). It doesn’t require repeating all the clinical trials done for the original drug. Instead, it focuses on one critical thing: bioequivalence. That means the generic must get into your bloodstream at the same rate and to the same level as the brand. The FDA requires that the amount of drug absorbed (measured as AUC) and the peak concentration (Cmax) fall within 80% to 125% of the brand’s numbers. That’s not a wide gap - it’s tighter than the natural variation you’d see between two different batches of the same brand-name drug.
Think of it like two identical cars from the same factory. One is painted red, one is blue. They have the same engine, same fuel efficiency, same safety features. The color doesn’t change how they drive. That’s what generics are.
Why Do the Labels Look So Different?
Here’s where confusion starts. Brand-name drugs have names you recognize - Lipitor, Zoloft, Ozempic. These are trademarked brand names. Generic drugs can’t use those names. So they use the chemical name of the active ingredient: atorvastatin, sertraline, semaglutide.
But it’s not just the name. The FDA requires generic labels to match brand-name labels in every important way: indications, dosage instructions, warnings, side effects, contraindications. The language about heart attacks, liver damage, or pregnancy risks is identical. You’re not missing out on safety info.
The only place labels can differ is in the inactive ingredients - things like fillers, dyes, and coatings. These don’t affect how the drug works. But because of trademark laws, generics must look different from the brand. That’s why your blue pill yesterday is now a white oval. It’s not a different drug. It’s just a different manufacturer.
Some patients get nervous when the pill changes color or shape. A University of Michigan study found 12% of patients initially worried they got the wrong medication. But when pharmacists explained what was happening, those fears dropped almost to zero. The pill’s appearance has zero effect on how it works.
How Much Do You Actually Save?
The savings aren’t small. They’re massive. In 2023, generic drugs made up 90% of all prescriptions filled in the U.S., but only 25% of total drug spending. That means for every $100 spent on prescriptions, $75 went to brand-name drugs - even though most people were taking generics.
Take atorvastatin. The brand-name Lipitor cost around $375 a month in early 2023. The generic? At Walmart, it was $4. That’s a 99% drop. For people on Medicare or without insurance, that difference means the difference between taking the drug and skipping doses.
From 2007 to 2016, generic drugs saved the U.S. healthcare system $1.67 trillion. In 2023 alone, they saved $313 billion. That’s not a guess. That’s from the Congressional Budget Office. These savings keep clinics open, lower insurance premiums, and let people afford their medications long-term.
And the FDA says 90% of generics cost less than $10 a month. For many, it’s under $5. That’s cheaper than a coffee.
Are There Any Exceptions?
Yes. And it’s important to know them.
Some drugs have a narrow therapeutic index (NTI). That means even tiny changes in blood levels can cause harm - either the drug doesn’t work, or it becomes toxic. The FDA specifically calls out warfarin (a blood thinner), levothyroxine (for thyroid), and phenytoin (for seizures) as drugs where substitution needs extra care.
For these, doctors and pharmacists often recommend sticking with the same manufacturer. Switching between different generic versions of levothyroxine, for example, can cause your TSH levels to shift. That’s not because generics are bad. It’s because the body is sensitive to tiny changes in these specific drugs. The solution? Don’t switch manufacturers unless your doctor says so. And get your blood tested after any switch.
There are also complex drugs that are harder to copy. Insulins, monoclonal antibodies, and auto-injectors like EpiPens aren’t simple pills. They’re biologics or drug-device combos. True generics for these don’t exist yet. Instead, we have biosimilars - similar, but not identical. They’re still cheaper than the brand, but not as straightforward as a generic pill.
What Do Real Patients Say?
People who’ve switched to generics report overwhelmingly positive experiences. On Drugs.com, generic atorvastatin has a 6.6 out of 10 rating from over 1,800 reviews. Lipitor, the brand, has a 6.3 from fewer than 900. That’s not a difference - it’s the same.
One pharmacist on Reddit, u/MedReviewExpert, said they’ve switched thousands of patients from brand to generic with zero issues - except for a few on levothyroxine, where they monitored thyroid levels closely.
A Kaiser Permanente survey in 2022 found that 78% of patients said generic cost savings helped them stick to their meds. That’s huge. Many people skip doses because drugs are too expensive. Generics fix that.
And studies back it up. A 2021 JAMA Internal Medicine study tracked 2 million patients on heart medications. No difference in outcomes between generic and brand. A 2023 review in BMJ Open looked at 47 clinical trials. Same result.
How Do You Know If a Generic Is Approved?
The FDA’s Orange Book is the official list of all approved drugs - brand and generic. It’s online, free, and updated daily. Each drug is listed with an “A” rating if it’s therapeutically equivalent to the brand. That’s your green light.
If you’re unsure, ask your pharmacist. They can pull up the Orange Book rating on the spot. Or check the FDA’s new Generic Drug Program Dashboard, launched in 2023, which shows real-time approval status for new generics.
Also, watch for the “AB” rating. “A” means equivalent. “B” means not equivalent - rare, but it happens. If you see a “B,” ask why. It’s not normal.

Can Your Pharmacist Switch Your Drug Without Asking?
In 49 out of 50 U.S. states, yes. Pharmacists can substitute a generic unless the doctor writes “Dispense as Written” on the prescription. That’s standard practice. It’s not a trick. It’s designed to save money and increase access.
Doctors are on board too. The American Medical Association found that 94% of physicians feel comfortable prescribing generics. Most prefer them - because they know the data.
But if you’re worried about switching, talk to your doctor. You can ask them to write “Dispense as Written” if you’ve had a bad experience or are on a sensitive medication. But don’t assume you need to - most people don’t.
What’s Changing Now?
The FDA is pushing hard to approve more complex generics - things like inhalers, topical creams, and injectables that were once too hard to copy. In 2022, they approved 79 complex generics - up 22% from the year before. The first generic version of Ozempic (semaglutide) was approved in September 2023. That’s a game-changer for millions.
The Generic Drug User Fee Amendments (GDUFA III), effective since October 2022, cut review times for priority generics to 10 months. That means faster access to cheaper drugs.
And the market is growing. Teva, Sandoz, and Amneal are the big players. But with $268 billion in brand-name drugs set to lose patent protection between 2023 and 2028, more companies are jumping in. Expect more generics, more savings, and more options.
Bottom Line: Take the Generic
There’s no reason not to. For 99% of prescriptions, the generic is just as safe, just as effective, and far cheaper. The label differences are about trademarks, not medicine. The pill color change? It’s the law - not a warning.
If you’re on warfarin, levothyroxine, or phenytoin, talk to your doctor before switching manufacturers. But don’t avoid generics because of fear. The science is clear. The data is solid. The savings are real.
Next time you get a prescription, ask: Is there a generic? If the answer is yes, say yes. Your wallet - and your health - will thank you.
Are generic drugs as safe as brand-name drugs?
Yes. The FDA requires generic drugs to meet the same strict standards for safety, strength, quality, and purity as brand-name drugs. They must contain the same active ingredient, in the same amount, and work the same way in the body. The FDA monitors both types of drugs through the same post-market safety system. There is no evidence that generics are less safe.
Why do generic pills look different from brand-name pills?
U.S. trademark laws require generic drugs to look different from the brand-name version - different color, shape, size, or markings. This prevents confusion and protects the brand’s intellectual property. But these visual differences don’t affect how the drug works. The active ingredient and dosage are identical. The change in appearance is purely cosmetic and legal, not medical.
Can I trust generics for chronic conditions like high blood pressure or diabetes?
Absolutely. Large studies, including one published in JAMA Internal Medicine tracking 2 million patients, show no difference in effectiveness between generic and brand-name drugs for heart disease, diabetes, and other chronic conditions. Generic atorvastatin, metformin, and lisinopril work just as well as their brand-name versions. The FDA requires bioequivalence testing to prove this before approval.
Why are generics so much cheaper?
Generics are cheaper because they don’t need to repeat expensive clinical trials. The original drug company already paid for the research, development, and marketing. Generic manufacturers only need to prove their version works the same way - which costs far less. They also face competition from multiple companies making the same drug, which drives prices down. The result? Generics cost 80-85% less on average.
What should I do if I think my generic drug isn’t working?
First, don’t assume it’s the generic. Many factors can affect how you feel - stress, diet, other medications, or even changes in your condition. If you’re concerned, talk to your doctor or pharmacist. For most drugs, switching between generic manufacturers won’t cause problems. But for narrow therapeutic index drugs like warfarin or levothyroxine, your provider may check your blood levels to make sure you’re still in the right range. Never stop taking your medication without professional advice.
Is there a list of approved generic drugs I can check?
Yes. The FDA’s Orange Book is the official public database of all approved drug products, including generics. It shows which generics are rated as therapeutically equivalent (marked with an “A”) to the brand-name drug. You can search it for free at the FDA’s website. Pharmacists also use it daily to verify substitutions.
Can pharmacists switch my brand-name drug to a generic without my permission?
In 49 U.S. states, yes - unless your doctor writes “Dispense as Written” on the prescription. This is legal and common. Pharmacists are trained to make these substitutions to save money and improve access. If you prefer the brand, you can ask for it, but you may pay more. If you’re unsure, ask your pharmacist to explain why they switched.
Are all generics made in the same place?
No. Generic drugs are made in facilities around the world, including the U.S., India, China, and Europe. The FDA inspects all facilities - domestic and foreign - that make drugs sold in the U.S. They use the same standards for safety and quality. A generic made in India is held to the same FDA standards as one made in New Jersey. The location doesn’t determine safety - the inspection process does.




Write a comment
Your email address will be restricted to us