Tag: generic drugs
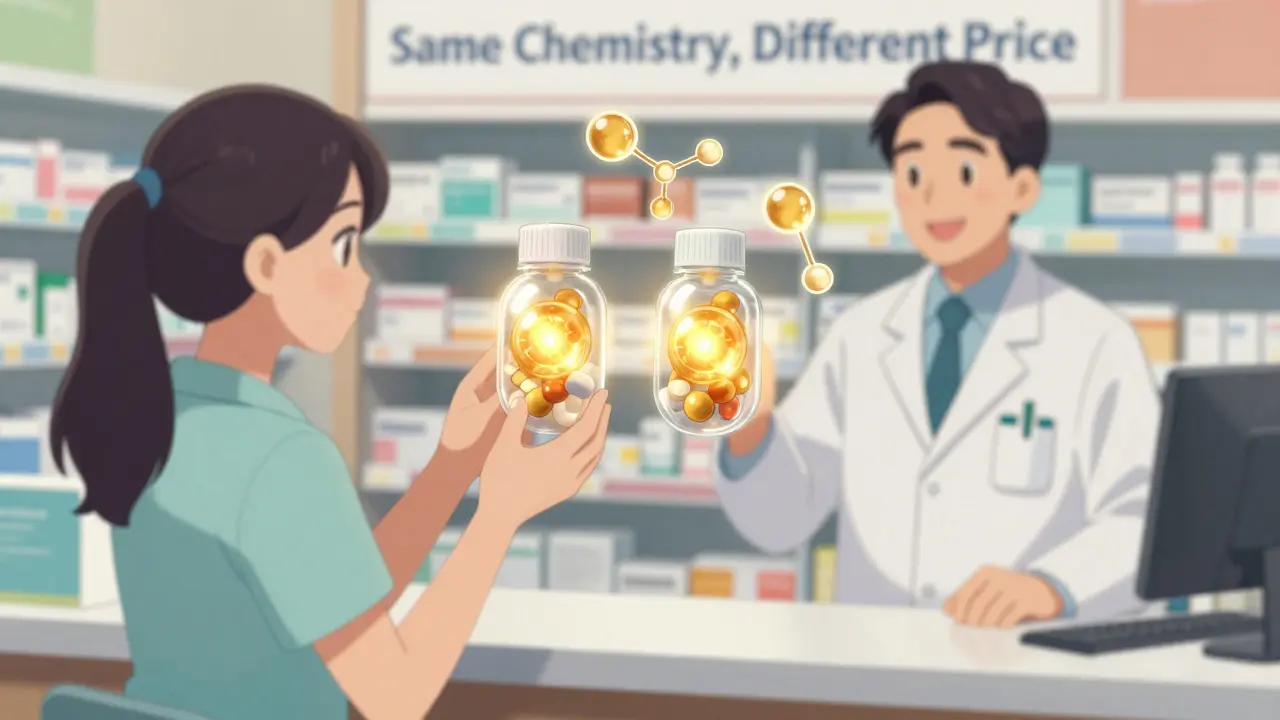
Drug Interactions: Same Risk for Generic and Brand Medications
Generic and brand-name drugs have the same active ingredients and identical interaction risks. Scientific evidence and regulatory standards confirm that switching to generics doesn't increase drug interaction dangers - and can save you money without sacrificing safety.
Quality concerns: when clinicians question generic manufacturing
Clinicians are raising alarms about the quality of generic drugs made overseas, especially in India and China. Studies show higher rates of adverse events, supply chain risks, and inspection gaps-raising urgent questions about safety, transparency, and the future of affordable medicine.
Generic vs Brand Name Drugs: What the Label Really Tells You and Why They Work the Same
Generic drugs are just as safe and effective as brand-name drugs, but cost up to 95% less. Learn how the FDA ensures therapeutic equivalence, why labels look different, and when to be cautious - with real data and patient insights.
Hospital Formularies: How Systems Choose Generic Drugs
Hospital formularies systematically select generic drugs based on clinical evidence, safety, and cost-effectiveness. Learn how P&T committees make these decisions and why generics dominate hospital prescribing.
Cmax and AUC in Bioequivalence: What Peak Concentration and Total Exposure Really Mean
Cmax and AUC are the two key pharmacokinetic measures used to prove generic drugs work the same as brand-name versions. Learn what they mean, why both are required, and how regulators ensure safety and effectiveness.
How to Save Money with Generics Without Sacrificing Safety
Learn how to save hundreds or even thousands on prescriptions by using generic medications safely. Discover which drugs are safe to switch, which ones need caution, and how to avoid hidden risks-all backed by FDA data and real patient results.
Cost-Effectiveness Analysis: Measuring the Real Value of Generic Drugs
Cost-effectiveness analysis reveals that the biggest savings in generic drugs come not from switching from brand to generic-but from swapping expensive generics for cheaper, equally effective alternatives. Learn how pricing distortions and outdated models are costing billions.
How Doctors Around the World View Generic Medicines
Doctors around the world have very different views on generic medications. In Europe and Japan, they're policy-driven. In Asia, they're essential. In the U.S., they're widespread but under scrutiny. Here's how global systems shape provider attitudes.